求助这个轨道的电子占据数分析方法
小弟我想做一个类似下面这样的轨道图1:这是一个催化剂活化氢气的过渡态,通过把过渡态分成催化剂的一部分和氢气另一部分,总共两个部分,然后做出分别作出这两个部分的HOMO和LUMO轨道图,这些我知道用高斯做单点就可以得到,但是他还可以算出这两个独立部分的HOMO和HOMO在过渡态中的电子分布,比如H2的HOMO中电子为1.393转移了0.683e到催化剂的LUMO,而催化剂的HOMO电子为1.714e转移0.284到氢气的反键轨道,我想知道这样的分析是怎么做的,我尝试过用Multiwfn的CDA分解功能,但是这个功能是从配合物轨道的角度去算每个每个配合物轨道的donation和back donation,我想从这两个片段出发,而且只要知道片段的HOMO和LUMO电子分布就可以了,就和图1是一样的,查了各种资料,还是不会做,请教大家,献上我的金币,回复的正确的我还给你追加金币。下面我把这个文章的这部分内容贴上,我再放上这个文章的原文,真的很想知道怎么做的,谢谢谢谢!
MO Analysis of TS2/3. To clarify the reason why H−H σ-
bond cleavage leads to the formation of two positively charged
hydrogen atoms, we performed MO analysis of TS2/3.
Generally, MOs of a total system AB can be represented by a
linear combination of MOs of fragments A and B; see eq 1,28
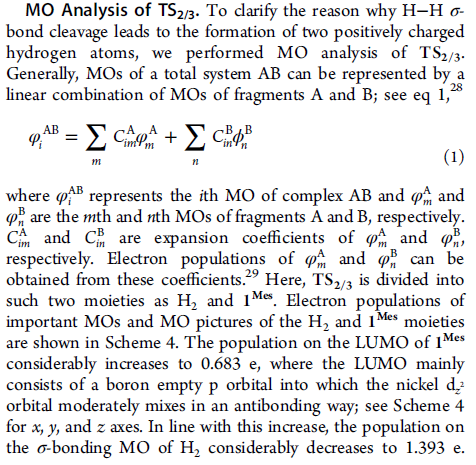
φi = ΣC φ + ΣC ϕ
m
im m
n
in n
AB A A B B
(1)
where φi
AB represents the ith MO of complex AB and φm A and
φn
B are the mth and nth MOs of fragments A and B, respectively.
Cim
A and Cin
B are expansion coefficients of φm A and φn
B,
respectively. Electron populations of φm A and φn
B can be
obtained from these coefficients.29 Here, TS2/3 is divided into
such two moieties as H2 and 1Mes. Electron populations of
important MOs and MO pictures of the H2 and 1Mes moieties
are shown in Scheme 4. The population on the LUMO of 1Mes
considerably increases to 0.683 e, where the LUMO mainly
consists of a boron empty p orbital into which the nickel dz2
orbital moderately mixes in an antibonding way; see Scheme 4
for x, y, and z axes. In line with this increase, the population on
the σ-bonding MO of H2 considerably decreases to 1.393 e.These results clearly indicate that CT substantially occurs from
the σ-bonding MO of H2 to the LUMO of 1Mes. Furthermore,
the population of the σ*-antibonding MO of H2 somewhat
increases to 0.284 e. Accordingly, the electron population on
the highest occupied molecular orbital (HOMO) of 1Mes
somewhat decreases to 1.714 e, where the HOMO mainly
consists of the nickel dx2−y2 orbital. These population changes
indicate that CT occurs from the doubly occupied nickel dx2−y2
orbital to the H2 σ*-antibonding MO. Both of these CTs
contribute to a weakening of the strong H−H σ-bond.
However, it is noted that the former CT is much larger than
the latter one, indicating that CT from H2 to 1Mes plays a
dominant role in the H−H σ-bond cleavage process. This is
consistent with natural bond order analysis along the IRC that
H−H σ-bond cleavage leads to the formation of two positively
charged hydrogen atoms. When the boron atom is replaced by
the CH group, the former CT becomes much weaker, which
leads to the formation of two much less negatively charged
hydrogen atoms (−0.06 and −0.07 e, respectively). The higher
ΔG°⧧ value of the H2 activation reaction mediated by 1−CH
also confirms the importance of CT from H2 to 1Mes. These
results indicate that the borane ligand facilitates H−H σ-bond
cleavage by inducing large CT from H2 to 1Mes.
1.png
2.png

3.png




 京公网安备 11010802022153号
京公网安备 11010802022153号
,